Choosing the right Clinical Data Management (CDM) company is a critical decision for any clinical trial. The success of the trial, the integrity of the data, and ultimately the safety and efficacy of the medical products being tested depend significantly on the quality of the CDM services. This article will guide you through the essential factors to consider when selecting a Clinical Data Management company.
Understanding Clinical Data Management
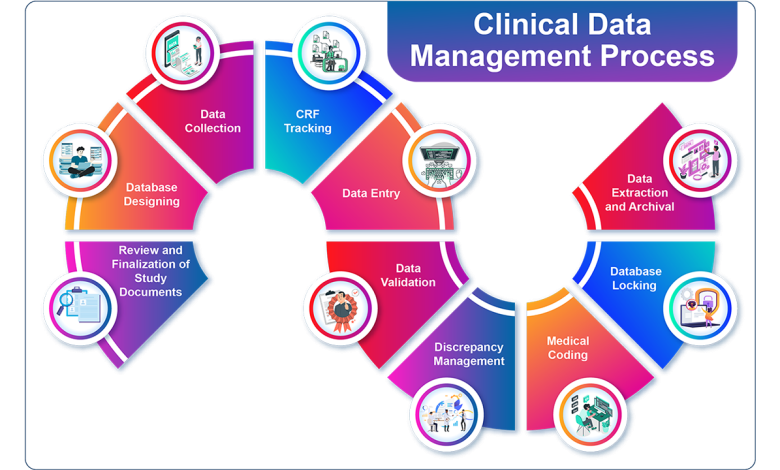
Before diving into the selection process, it’s crucial to understand what Clinical Data Management entails. CDM involves the collection, cleaning, and management of data generated during clinical trials. The goal is to ensure data quality, integrity, and compliance with regulatory requirements. Key activities include:
- Data Collection: Designing case report forms (CRFs), electronic data capture (EDC) systems.
- Data Validation: Ensuring accuracy and consistency through validation checks.
- Data Cleaning: Identifying and resolving discrepancies or errors.
- Data Coding: Standardizing data using medical dictionaries.
- Database Lock: Finalizing the database for analysis.
Key Factors to Consider
1. Experience and Expertise
When choosing a CDM company, their experience and expertise in handling clinical trial data are paramount. Consider the following:
- Track Record: Look at their history of managing similar trials.
- Therapeutic Area Expertise: Ensure they have experience in the specific therapeutic area of your trial.
- Regulatory Knowledge: Their familiarity with regulatory requirements (FDA, EMA, etc.) and guidelines (ICH-GCP).
2. Technology and Tools
The technology a CDM company uses can significantly impact the efficiency and quality of data management. Assess their:
- Electronic Data Capture (EDC) Systems: These should be user-friendly, reliable, and compliant with regulatory standards.
- Data Management Software: Ensure they use advanced, validated software for data cleaning, validation, and coding.
- Data Security: Their systems should have robust security measures to protect sensitive data.
3. Quality Assurance
Quality assurance processes are critical to maintaining data integrity. Evaluate the company’s:
- Standard Operating Procedures (SOPs): Well-documented SOPs for all CDM activities.
- Quality Control Measures: Regular audits, validation checks, and quality assessments.
- Certifications: Certifications like ISO 9001 or 21 CFR Part 11 compliance can indicate high-quality standards.
4. Flexibility and Scalability
Clinical trials can vary significantly in size and complexity. A good CDM company should be able to scale their services according to your needs. Consider their:
- Resource Availability: Ability to allocate sufficient resources to your project.
- Adaptability: Willingness to customize their services to fit your specific requirements.
- Global Reach: Capability to manage multinational trials if required.
5. Communication and Collaboration
Effective communication and collaboration are crucial for the smooth operation of a clinical trial. Evaluate the company’s:
- Project Management Skills: Experience in managing timelines, budgets, and deliverables.
- Communication Channels: Availability of clear and consistent communication channels.
- Client Testimonials: Feedback from previous clients regarding their communication and collaboration.
6. Cost Considerations
While cost should not be the sole deciding factor, it is essential to consider your budget. Compare the pricing structures of different companies and ensure there are no hidden costs. Look for:
- Transparent Pricing: Clear breakdown of costs.
- Value for Money: Balance between cost and the quality of services provided.
Steps to Make an Informed Decision
- Define Your Needs: Clearly outline the scope, scale, and specific requirements of your clinical trial.
- Research Potential Companies: Create a shortlist based on recommendations, online reviews, and initial assessments.
- Request Proposals: Ask for detailed proposals from the shortlisted companies, including case studies and references.
- Evaluate Proposals: Compare the proposals based on the key factors discussed above.
- Conduct Interviews: Arrange meetings or calls with the top candidates to discuss your project in detail.
- Check References: Contact previous clients to get insights into their experiences.
- Make Your Decision: Choose the company that best aligns with your needs, budget, and expectations.
Conclusion
Choosing the right Clinical Data Management company is a strategic decision that can significantly influence the success of your clinical trial. By carefully considering factors such as experience, technology, quality assurance, flexibility, communication, and cost, you can make an informed choice that ensures high-quality data management and contributes to the overall success of your research.